The purpose of the dgfr package is to enable the visualization of sequence diversity within gene/protein families. It is also possible to determine the optimal number of clusters for grouping members of gene families based on sequence similarity and to calculate the mean/median similarity inside each cluster.
Installation
Before proceeding to the installation of the dgfr package, you must install the following dependencies:
if (!requireNamespace("BiocManager", quietly = TRUE)) {
install.packages("BiocManager")
}
BiocManager::install("Biostrings", dependencies = TRUE)Now you can install the dgfr package using the following command:
How to use?
- First, retrieve the FASTA sequences of the gene family being studied from the database of your choice. The fasta file must be loaded inside R using the Biostrings::readAAStringSet() in case of protein sequences or Biostrings::readDNAStringSet() in case of nucleotide sequences.
tcmuc_fasta <- Biostrings::readAAStringSet("data-raw/tcmuc_fasta")If you prefer, you can utilize a sample dataset included in the package named fasta, which comprises 75 protein sequences. In this instance, I will employ the complete set of proteins tcmuc_fasta.
data(fasta)- The object containing your fasta should be provided to the first function named
get_alignment_score, then you should also provide the type o sequence “nuc” for nucleotide or “prot” for protein, the alignment method (“global” or “local”) and the number of cores to be used in the analysis. The score is calculated using the following formula:
- algn_seq1seq2 / (sqrt(algn_seq1seq1) * sqrt(algn_seq2seq2))
This formula was first proposed by Bleakley and Yamanashi and is a way to normalize the score by the length of the alignment. The output will be a dataframe containing the score of each pairwise alignment among all sequences provided.
score_file <- dgfr::get_alignment_score(fasta = tcmuc_fasta,
type = "prot",
alignment_method = "global",
cores = 4)
#> [1/6] Loading substitution matrix...
#> [2/6] Loading fasta file...
#> [3/6] Creating all possible sequence combinations...
#> [4/6] Performing pairwise alignment for prot sequences. This might take a while...
#> [5/6] Finishing up...
#> [6/6] Pairwise alignment is done.- The output of the first function of the package, should be provided to the second function called
create_distance_matrix. This function calculates the average Euclidian distance between the score of each pairwise alignment.
distance_matrix <- dgfr::create_distance_matrix(score_file = score_file)- After generating the distance matrix, we will use the
get_variancefunction to access the variability explained by each axis. This requires the provision of the file containing the distance matrix. In general, a cumulative value of 70% or higher for the axis is considered adequate for explaining the data’s variance.
variance <- dgfr::get_variance(distance_matrix = distance_matrix)
variance |> dplyr::select(1:3)
#> # A tibble: 1 × 3
#> `1` `2` `3`
#> <dbl> <dbl> <dbl>
#> 1 81.7 10.5 3.34- The subsequent required step consists of a multidimensional scaling (MDS) of the data matrix. The output will consist of the name of the sequence and its position on axes 1 and 2.
dim_reduced_file <- dgfr::dim_reduction(distance_matrix = distance_matrix)
head(dim_reduced_file)
#> axis_1 axis_2
#> TcYC6_0150500-RA-p1 0.01410299 0.08506485
#> TcYC6_0150510-RA-p1 -1.10954550 0.30707240
#> TcYC6_0150520-RA-p1 -0.97152461 0.20716940
#> TcYC6_0150530-RA-p1 -1.13936354 0.29036688
#> TcYC6_0150540-RA-p1 -0.73347654 0.01002143
#> TcYC6_0150550-RA-p1 0.43034170 0.19684138- A possible functionality of the dgfr package is to determine the optimal number of clusters in the dataset. You must provide the dimension-reduced file and, if desired, the minimum and maximum number of clusters to be identified. If you do not provide these values, the script will automatically search between 2 and 20 clusters.
kmeans_output <- dgfr::kmeans_clustering(dim_reduced_file = dim_reduced_file)Finally, you will have a table containing the name of each sequence, its position on axes 1 and 2, and the cluster to which the sequence belongs.
head(kmeans_output)
#> name axis_1 axis_2 cluster
#> 1 TcYC6_0150500-RA-p1 0.01410299 0.08506485 1
#> 2 TcYC6_0150510-RA-p1 -1.10954550 0.30707240 1
#> 3 TcYC6_0150520-RA-p1 -0.97152461 0.20716940 1
#> 4 TcYC6_0150530-RA-p1 -1.13936354 0.29036688 1
#> 5 TcYC6_0150540-RA-p1 -0.73347654 0.01002143 1
#> 6 TcYC6_0150550-RA-p1 0.43034170 0.19684138 1- The next function, allows you to visualize the sequences and its cluster in a tree-like structure. You must provide the
kmeans_clusteringfunction output and thecreate_distance_matrixoutput. The output will be a plot containing the sequences and its cluster.
tree <- dgfr::create_tree(distance_matrix = distance_matrix,
kmeans_output = kmeans_output)
tree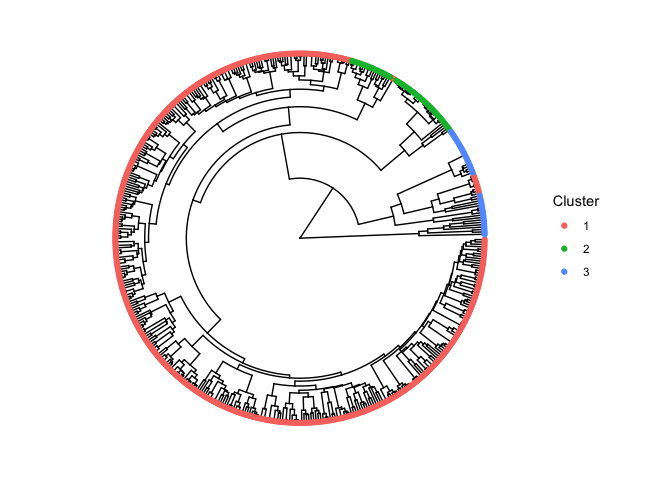
- Finally, you can access the mean/median similarity among each cluster using the function
similarity_cluster. This function takes as argument thekmeans_clusteringfunction output, theget_alignment_scoreoutput and you must also choose between two possible visualizations output. The “table” output returns a table containing the mean and median similarity among all clusters and the number of genes inside each cluster.
dgfr::similarity_cluster(score_file = score_file,
kmeans_output = kmeans_output,
output_type = "table")
#> # A tibble: 3 × 4
#> cluster mean_similarity median_similarity number_of_genes
#> <chr> <dbl> <dbl> <int>
#> 1 cluster_1 0.509 0.503 391
#> 2 cluster_2 0.756 0.707 49
#> 3 cluster_3 0.434 0.260 36The “plot” output returns a plot containing a violin plot highlighting the distribution of similarity within each cluster.
dgfr::similarity_cluster(score_file = score_file,
kmeans_output = kmeans_output,
output_type = "plot")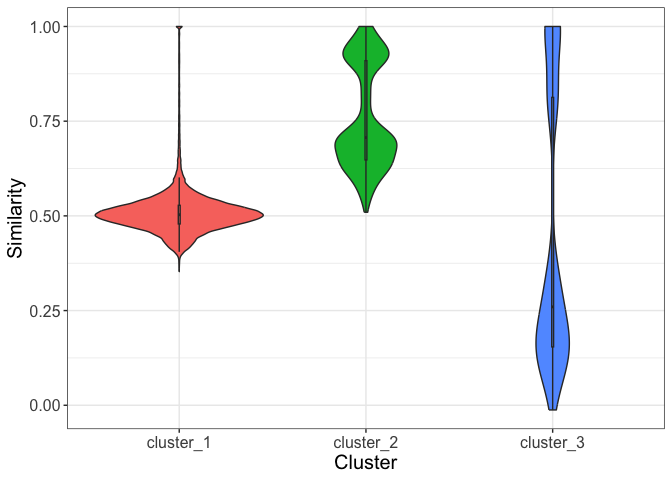
Data visualisation suggestions
All visualization suggestions presented here were created using the ggplot2 package. However, you are allowed to use your preferred package or tool to visualize the obtained data.
Visualizing gene family dispersion
The simplest form of visualization is the scatter plot of sequences from a particular gene family, where each point represents a sequence, and the distance between two points is proportional to the similarity of these sequences.
kmeans_output |>
ggplot2::ggplot(
ggplot2::aes(x = axis_1,
y = axis_2)) +
ggplot2::geom_point(size = 3.5, alpha = 0.3) +
ggplot2::theme_bw() +
ggplot2::labs(x = round(variance$`1`, 2),
y = round(variance$`2`, 2))
Identifying particular sequences within the gene family
The second suggested plot is similar to the first, but it allows for the highlighting of sequences of interest. In addition to ggplot2, gghighlight and ggrepel are required to perform this analysis.
seq_name <- kmeans_output |>
dplyr::sample_n(5) |>
dplyr::select(name) |>
dplyr::pull()
kmeans_output |>
ggplot2::ggplot(
ggplot2::aes(x = axis_1,
y = axis_2)) +
ggplot2::geom_point(size = 3.5, alpha = 0.3) +
ggplot2::theme_bw() +
ggplot2::labs(x = round(variance$`1`, 2),
y = round(variance$`2`, 2)) +
gghighlight::gghighlight(name %in% seq_name) +
ggrepel::geom_text_repel(label = seq_name,
min.segment.length = 0,
seed = 42,
box.padding = 0.5)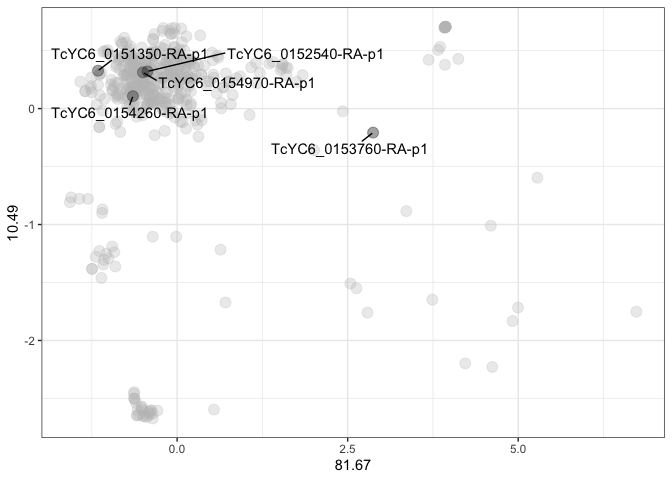
Identifying the number of clusters within the gene family
In this third visualization suggestion, the gene family is divided into an optimal number of groups, each of which represented by a different color.
kmeans_output |>
dplyr::mutate(cluster = as.character(cluster)) |>
ggplot2::ggplot(
ggplot2::aes(x = axis_1,
y = axis_2,
color = cluster)) +
ggplot2::geom_point(size = 3.5, alpha = 0.3) +
ggplot2::theme_bw() +
ggplot2::labs(x = round(variance$`1`, 2),
y = round(variance$`2`, 2))
Aggregating and visualizing RNAseq data
Another functionality is to integrate RNAseq data to visualize the expression and dispersion of the gene family in the scatter plot. The size of the points is proportional to the gene expression level of each family member, with small points representing genes with low expression and large points representing genes with high expression.
kmeans_out_l2fc <- kmeans_output |>
dplyr::mutate(l2fc = runif(476, min = 0.1, max = 4))
kmeans_out_l2fc |>
dplyr::mutate(cluster = as.character(cluster)) |>
ggplot2::ggplot(
ggplot2::aes(x = axis_1,
y = axis_2,
color = cluster)) +
ggplot2::geom_point(size = kmeans_out_l2fc$l2fc, alpha = 0.3) +
ggplot2::theme_bw() +
ggplot2::labs(x = round(variance$`1`, 2),
y = round(variance$`2`, 2))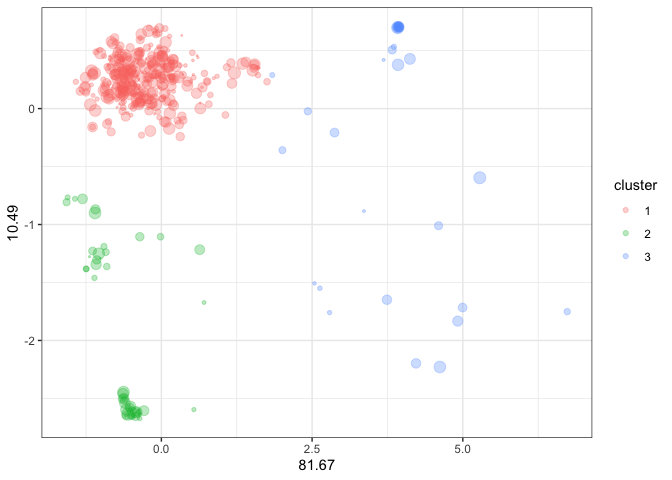
Acknowledgment
The development of this package would not have been possible without the knowledge shared by the instructors at curso-R, mainly Beatriz Milz, Caio Lente and Júlio Trecenti.
